Abstract
Introduction: Ibrutinib (I), the first irreversible inhibitor of Bruton's tyrosine kinase, and venetoclax (V), the first approved Bcl-2 inhibitor, have both improved outcomes in CLL in numerous clinical trials compared to CIT. Theoretically ibrutinib could be more effective in IGHV unmutated CLL as these cases appear to be more dependent on B-cell receptor signalling. Phase II and phase III randomised trials suggested that using I+V in combination could result in high proportions of MRD negativity, particularly in IGHV unmutated CLL. We therefore hypothesize that I+V may be more effective in IGHV unmutated CLL.
Methods: FLAIR is an ongoing, phase III, multicentre, randomised, controlled, open, parallel group trial for previously untreated CLL requiring therapy according to IWCLL. TP53 deleted patients were excluded from recruitment. FLAIR was adapted in July 2017 to add two arms, I monotherapy and I+V. I was given at 420mg/day for up to 6 years. In the I+V arm after the first 2 months of I, V was added with dose escalation to 400mg/day over the next month and then the combination was also given for up to a total of 6 years. In both arms the duration of therapy was defined by MRD status. PB and BM MRD was assessed at 9 months post-randomisation, PB MRD was then assessed at 12 months and every 6 months thereafter. When PB is MRD negative, it is repeated after 3 months and, if negative, PB and BM MRD are performed 3 months later. If both are MRD negative, then the initial MRD negative PB was considered the time to MRD negativity, and the planned duration of therapy will be twice that period. Therefore, the earliest a patient could stop therapy was 2 years post-randomisation. The primary endpoint for I vs I+V in FLAIR was to assess the rate of MRD eradication between I and I+V within 2 months post-randomisation. Key secondary endpoints presented here are IWCLL response and safety. MRD was assessed in a central laboratory by multiparameter flow cytometry and MRD negativity was defined as less than 1 CLL cell in 10,000 leucocytes. A formal interim analysis for MRD was performed once 50% of participants in the I and I+V arms had reached 2 years post-randomisation. For the interim analysis, if the p-value is less than 0.005, the result is statistically significant. Here we present the analysis of MRD negativity in peripheral blood and bone marrow within 2 years of continuous I + V combination analysed by CLL prognostic sub-groups.
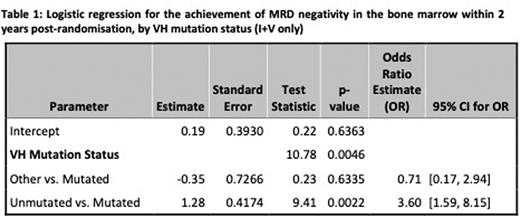
Results: 523 patients were randomised on a 1:1 basis between I and I+V. Here we report the interim analysis of the eradication of MRD in the first 274 patients randomized between I (n=138) and I+V (n=136) reaching 2 years post-randomisation from 83 UK Centres between 13/07/2017 and 15/03/2019. The data was locked on 2/8/2022. 72.1% were male, median age was 63 years (34.3% >65yo) and 40.9% were Binet Stage C. IGHV data was available for 256 (93.4%) patients with 48.2% IGHV unmutated, 45.3% IGHV mutated and 9.1% Subset 2. Hierarchical FISH testing revealed 16.1% 11q del, 19% trisomy 12, 21.9% normal and 36.9% 13q del; with 6.2% failed. The arms were well-balanced for disease variables with no significance differences. In the I+V arm, 51/64 (79.7%) IGHV unmutated were MRD-negative in the BM within 24 months compared with 31/55 (56.4%) IGHV mutated and 3/8 (37.5%) subset 2. At 9 months post-randomisation, 34/64 (53.1%) of IGHV unmutated patients were MRD-negative in the BM as compared to 19/55(34.5%) IGHV mutated and 3/8 (37.5%) subset 2. In the PB, the MRD-negative rates in IGHV unmutated patients were 35/64 (54.7%) at 9 month and 53/64 (82.8%) at 24 months post-randomisation respectively. The MRD-negative rates in the PB for IGHV mutated patients improved from 18/55 (32.7%) at 9 months post-randomisation to 35/55 (63.6%) at 24 months. The probability of achieving MRD-negativity within 24 months of I + V was higher in IGHV unmutated CLL with an odds ratio of 3.60 [95% CI: 1.59, 8.15] in favour of unmutated vs mutated CLL (p-value 0.0022). 26/31 (83.9%) of patients with 11q (ATM) deletion achieved MRD negativity at 2 years but 22/31 (71%) of these patients were IGHV unmutated compared to only 41/103 (39.8%) of those without ATM deletion.
Conclusion: Ibrutinib plus venetoclax leads to high MRD-negative rates in BM with 2 years of continuous therapy in both IGHV mutated and unmutated CLL. Patients with IGHV unmutated CLL are statistically more likely to achieve MRD negativity in the PB and BM with this combination as compared to IGHV mutated CLL.
Disclosures
Munir:Janssen, AstraZeneca, Alexion, Sobi, Novartis, Roche, Abbvie, Gilead: Honoraria; Janssen, AstraZeneca, Alexion, Abbvie, Novartis, Roche: Membership on an entity's Board of Directors or advisory committees. Bloor:Janssen: Consultancy, Honoraria, Other: Grant and personal fees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Grant and personal fees, Speakers Bureau. Pettitt:BMS/Celgene: Other: Grant and non financial support, Research Funding; Chugai: Other: Grant, personal fees and non financial support; Napp: Other: Grant and non financial support; Roche: Other: Grant, personal fees and non financial support, Research Funding; Gilead: Other: Grant, personal fees and non financial support; AstraZeneca: Other: Grant and non financial support; GSK/Novartis: Other: Grant and non financial support. Patten:Roche: Research Funding; Janssen: Honoraria; AbbVie: Honoraria; Gilead: Honoraria, Research Funding; AstraZeneca: Honoraria; Beigene: Honoraria. Forconi:Beigene: Speakers Bureau; AbbVie: Honoraria, Other: travel and accomodation, Speakers Bureau; Janssen: Honoraria, Other: travel and accomodation, Speakers Bureau; Astra-Zeneca: Honoraria, Speakers Bureau; BC platform: Consultancy. Schuh:Abbvie: Honoraria, Other: Personal fees; Roche: Honoraria, Other: Personal fees; Janssen: Honoraria, Other: Non-educational grant; AstraZeneca: Honoraria, Other: Non-educational grant; SERENOx: Membership on an entity's Board of Directors or advisory committees, Other: Founder of; Gilead: Honoraria, Other: Personal fees; Adaptive Biotechnology: Honoraria; Exact Sciences: Honoraria; Illumina: Other: In-kind contributions; Oxford Nanopore Technology: Other: In kind contributions. Fox:Janssen: Consultancy, Speakers Bureau; AbbVie: Consultancy; AstraZeneca: Consultancy; Atarabio: Consultancy; Celgene/BMS: Consultancy, Speakers Bureau; Beigene: Research Funding; GenMab: Consultancy; Gilead/Kite: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Roche: Consultancy, Other: Travel to scientific congress, Speakers Bureau; Morphosys: Consultancy; Ono: Consultancy; Takeda: Consultancy, Speakers Bureau. Kennedy:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Sheehy:Roche: Other: other fees; Janssen: Other: other fees. Howard:Roche Products Limited: Current Employment. Cairns:Celgene/BMS: Honoraria; Amgen: Research Funding; Takeda: Research Funding. Cwynarski:Roche, Takeda, Celgene/BMS, Atara, Gilead/KITE, Janssen, Incyte: Consultancy; BeiGene: Research Funding; Roche, Takeda, KITE/Gilead, Incyte: Speakers Bureau; Roche, Celgene/BMS, Takeda, KITE: Other: Travel to scientific congress. Paneesha:Gilead: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; AstraZeneca: Honoraria. Hillmen:Pharmacyclics: Other: Financial or material support, Research Funding; Janssen: Consultancy, Other: Financial or material support, Research Funding, Speakers Bureau; AbbVie: Consultancy, Other: Financial or material support, Research Funding, Speakers Bureau; Roche: Consultancy, Other: Financial or material support, Research Funding, Speakers Bureau; Acerta: Other: Financial or material support; Gilead: Other: Financial or material support, Research Funding; Alexion: Consultancy, Research Funding, Speakers Bureau; Apellis: Consultancy, Current Employment, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal